16+ Calculate Δssurrδssurr At The Indicated Temperature For A Reaction.
The change in entropy of the surroundings after a chemical reaction at constant pressure and temperature can be expressed by the formula ΔS surr -ΔHT where ΔS surr is. A C2H8 g 5 O2 g 3 CO2 g 4H2O g ΔH -2045 kJ the reaction takes place at 2 5oC.

A Numerical Model For Lithium Plasma Process In A Hybrid Microwave Ion Source Cui 2021 Contributions To Plasma Physics Wiley Online Library
18 Calculate Δssurrδssurr At The Indicated Temperature For A Reaction.

. The A H value for the reaction k0 g Hg 1 HgO s is -908 kJ. Calculate ΔSsurrΔSsurr at the indicated temperature for a reaction. Calculate the change in entropy for the surroundings ΔSsurr for the reaction at 250 C.
298 mathrm K ΔH rxn 385kJ298K. Calculate the entropy of the surroundings for the following reaction. Most standard Wikipedia Eng Bach Le October 11 2022 0 Comment Globalizethis aggregates.
The sign of ΔH is negative and. ΔHrxnΔHrxn 145 kJkJ. 4 Calculate ΔSsurr at the.
How much heat is released when. Web 1For the vaporization of benzene. We can convert this one into Joels so 307-0 Joels per mole.
10 calculate δssurrδssurr at the indicated temperature for a reaction. Calculate the change in. 3 Calculate the change in entropy that occurs in the system when 453 g of acetone C3H6O freezes at its melting point -948C.
Express your answer using two significant figures. Then it will be two Kelvin. That is equal to minus this one is 300.
What amount is received as payment in full on July 24. For a reaction to be spontaneous we must have ΔG 0. A reaction has a ΔHrxn542 kJ.
ΔHfus 569 kJmol. Delta S_ text surr ΔS surr. Delta H_ mathrm rxn circ-385 mathrm kJ.
ΔG ΔH T ΔS. A hot 70C lump of metal has a mass of 250 g and a specific heat of 025 calgC. Assume constant pressure and - 14592086.
ΔSsurr - ΔH sys T - -1267 kJ 398 K 318 kJK. At the indicated temperature for each reaction. Okay and temperature is in Kelvins plus Ah to 7315.
Cluster Based Single Molecule Magnets Springerlink
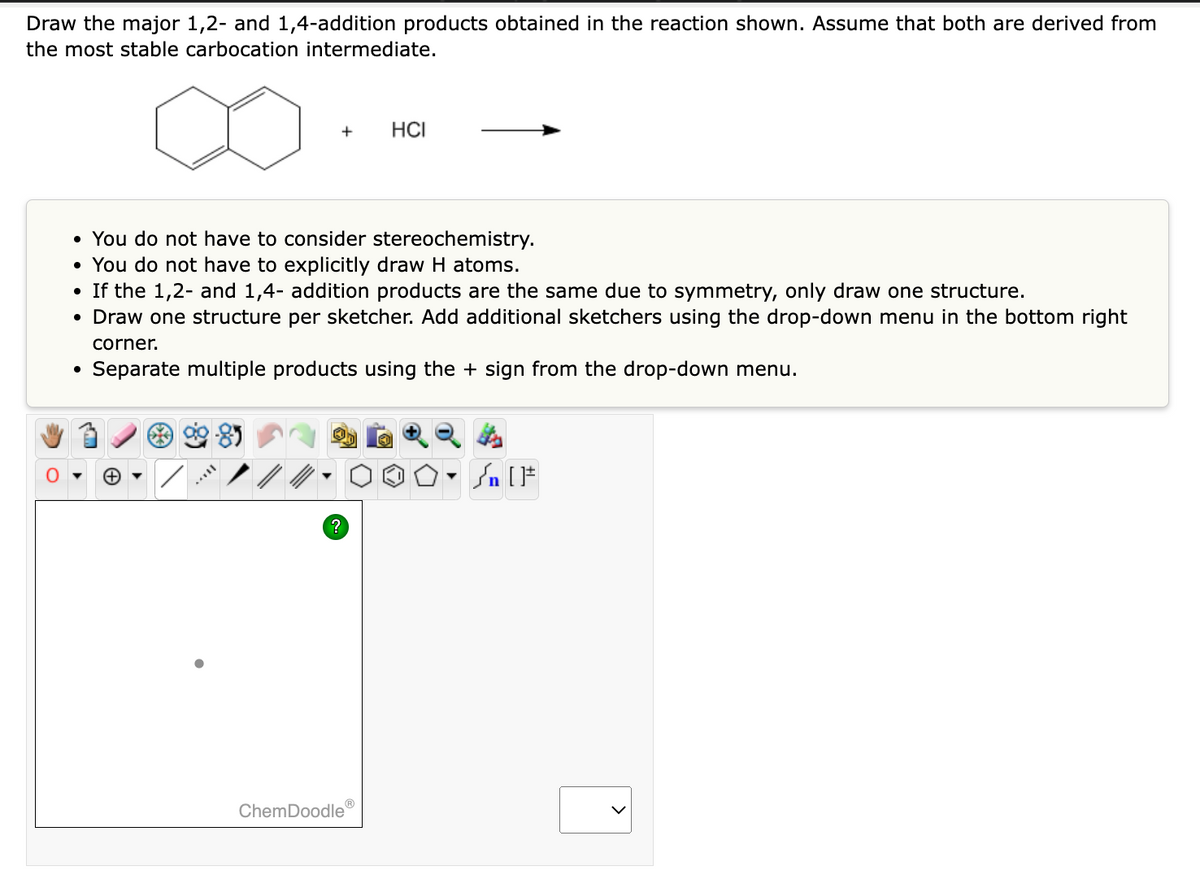
Answered Hci O Not Have To Consider Bartleby

For The Hydrolysis Of Methyl Acetate In Aqueous Solution The Above Tabulated Results Were Obtained A Show That It Follow Pseudo First Order Reaction As The Concentration Of Water Remains Constant B Calculate The
Ch 16 Therodinamics And Chemistry

Physical Chemistry 21 Understanding And Calculating Equilibrium Constants Slides And Tasks Teaching Resources

The Effect Of Temperature On Enzyme Activity Ocr A Level Biology Teaching Resources

Topological Connection Between Vesicles And Nanotubes In Single Molecule Lipid Membranes Driven By Head Tail Interactions Langmuir
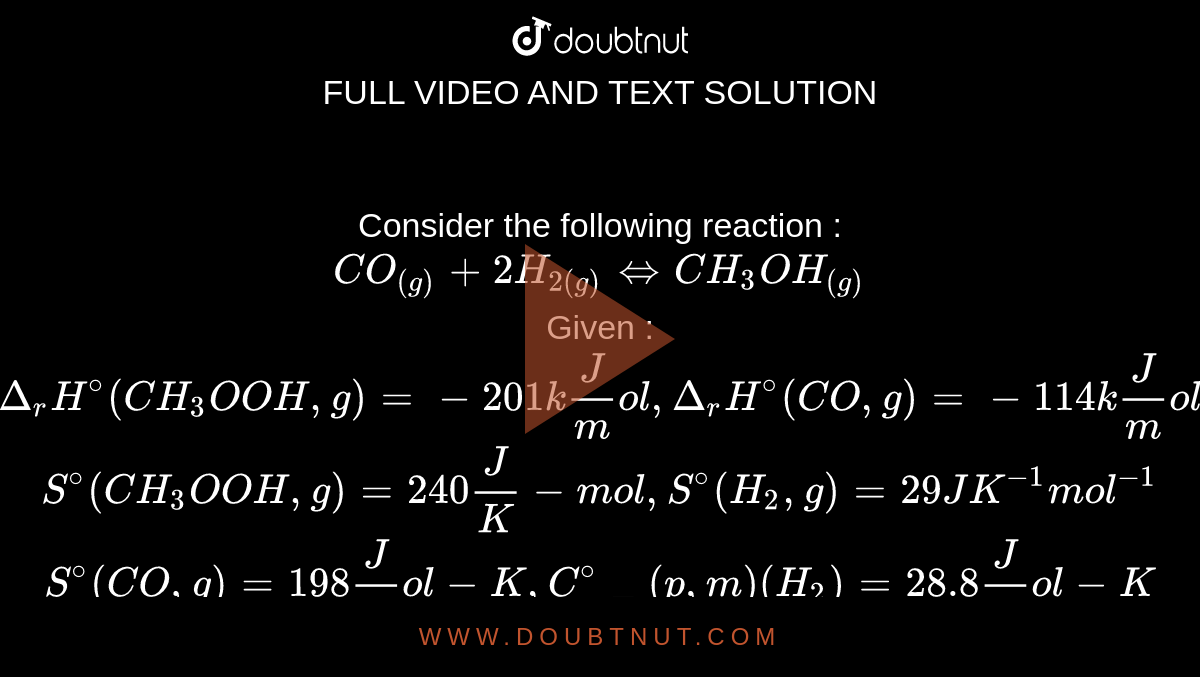
Consider The Following Reaction Co G 2h 2 G Iffch 3 Oh G Given Delta F H Ch 3 Oh G 201 Kj Mol Delta F H Co G 114 Kj Mol S Ch 3 Oh G 240 J K Mol S H 2 G 29 Jk 1 Mol 1 S Co G 198 J

Us20070099248a1 Monovalent Streptavidin Compositions Google Patents
Applications To Plasma Spectroscopy Springerlink

Uncertainty Estimates For Routine Temperature Data Sets Part Two Watts Up With That
Publications
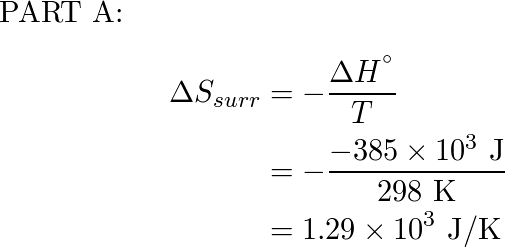
Calculate Delta S Text Surr At The Indicated Temper Quizlet

Chapter 8 Flashcards Quizlet
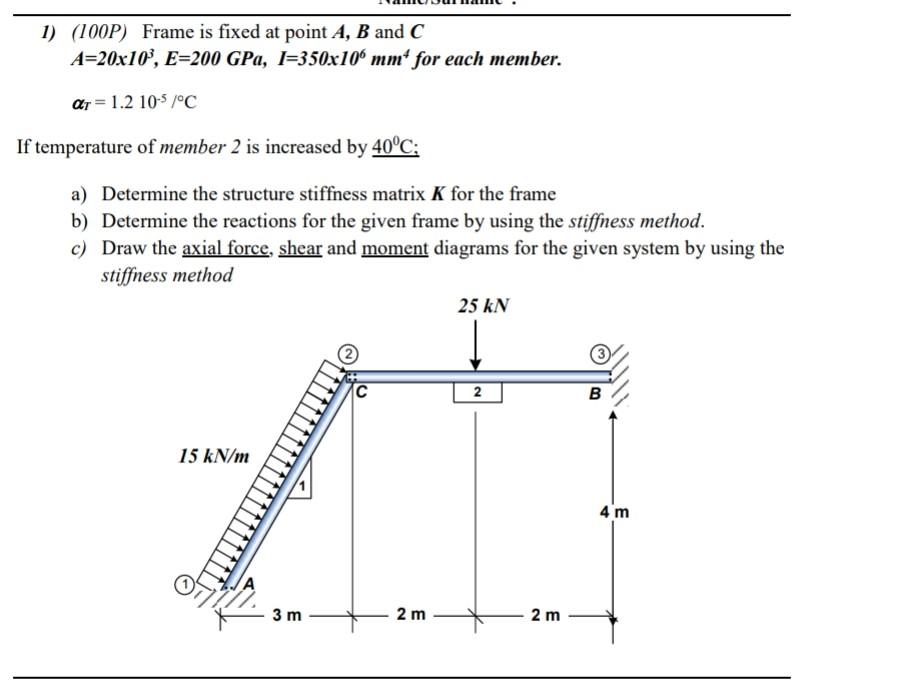
Solved 1 100p Frame Is Fixed At Point A B And C Chegg Com
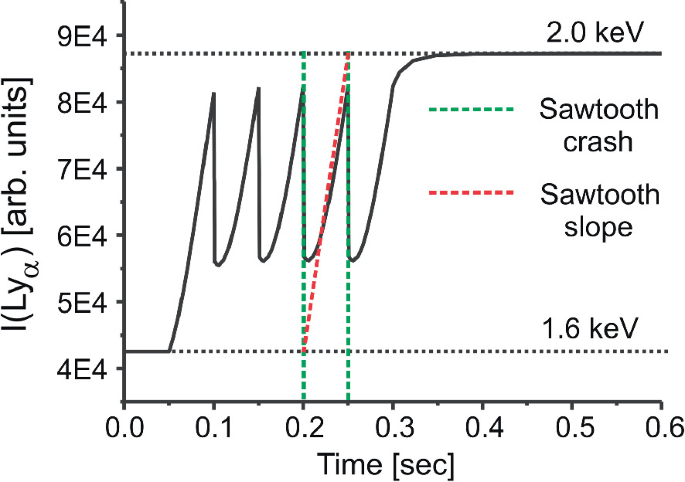
Applications To Plasma Spectroscopy Springerlink
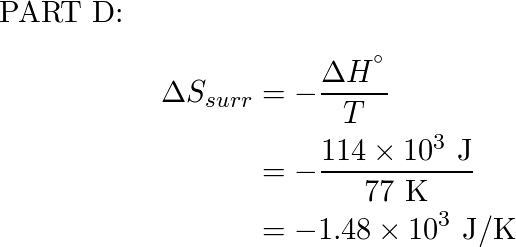
Calculate Delta S Text Surr At The Indicated Temper Quizlet